pasadena rose bowl freebie wamo
reboot normal horntail range
free instant win money games
local sex hookup
local sex offenders list usa
i want to fuck my apartment neighbor
british amateur granny swingers
magic the gathering uk singles
afk soren team
catalina 27 for sale craigslist
Lewis dot structures are diagrams that show the bonding between atoms and the lone pairs of electrons in a molecule. They are used to represent the valence electrons of atoms and how they are shared or transferred during chemical reactions. In this article, we will discuss the Lewis dot structure for NH2OH and how it can be determined. NH2OH, also known as hydroxylamine, is a chemical compound that consists of an amino group (NH2) and a hydroxyl group (OH). It is a colorless, crystalline solid that is used in various industrial processes, including the production of pharmaceuticals, rubber, and textiles. To determine the Lewis dot structure for NH2OH, we need to consider the valence electrons of each atom. Nitrogen (N) is in Group 15 of the periodic table and has five valence electrons. Hydrogen (H) is in Group 1 and has one valence electron, while oxygen (O) is in Group 16 and has six valence electrons. First, we start by placing the least electronegative atom, which is hydrogen, in the center. Hydrogen can only form one bond, so we put it in the center and surround it with dots to represent its valence electron. Next, we place the nitrogen atom next to the hydrogen atom and connect them with a single bond. Nitrogen has five valence electrons, so we place four dots around it. The remaining electron is placed on the nitrogen atom. Then, we place the oxygen atom next to the nitrogen atom and connect them with a single bond. Oxygen has six valence electrons, so we place two dots around it. The remaining four electrons are placed as lone pairs around the oxygen atom. Finally, we complete the structure by placing the remaining hydrogen atom next to the oxygen atom and connecting them with a single bond. Hydrogen has one valence electron, so we place it as a dot around the hydrogen atom. The final Lewis dot structure for NH2OH is as follows: H | N - O | | H H In this structure, the nitrogen atom is bonded to two hydrogen atoms and one oxygen atom. The oxygen atom is bonded to the nitrogen atom and one hydrogen atom. The lone pairs of electrons are represented by dots around the atoms. The Lewis dot structure for NH2OH helps us understand the bonding and electron distribution in the molecule. It shows that nitrogen forms three bonds and has one lone pair of electrons, oxygen forms two bonds and has two lone pairs of electrons, and hydrogen forms one bond and has no lone pairs of electrons. By understanding the Lewis dot structure, we can predict the geometry and polarity of the molecule. In the case of NH2OH, the molecule has a bent or V-shaped geometry due to the lone pairs of electrons on the nitrogen and oxygen atoms. The molecule is polar due to the difference in electronegativity between nitrogen and hydrogen, as well as oxygen and hydrogen. In conclusion, the Lewis dot structure for NH2OH is an important tool for understanding the bonding and electron distribution in the molecule. It helps us determine the geometry and polarity of the molecule, which are important factors in predicting its chemical properties and behavior.
NH2OH Lewis Structure: How to Draw the Lewis Structure for NH2OH .. ----- Steps to Write Lewis Structure for compounds like NH2OH ----- 1
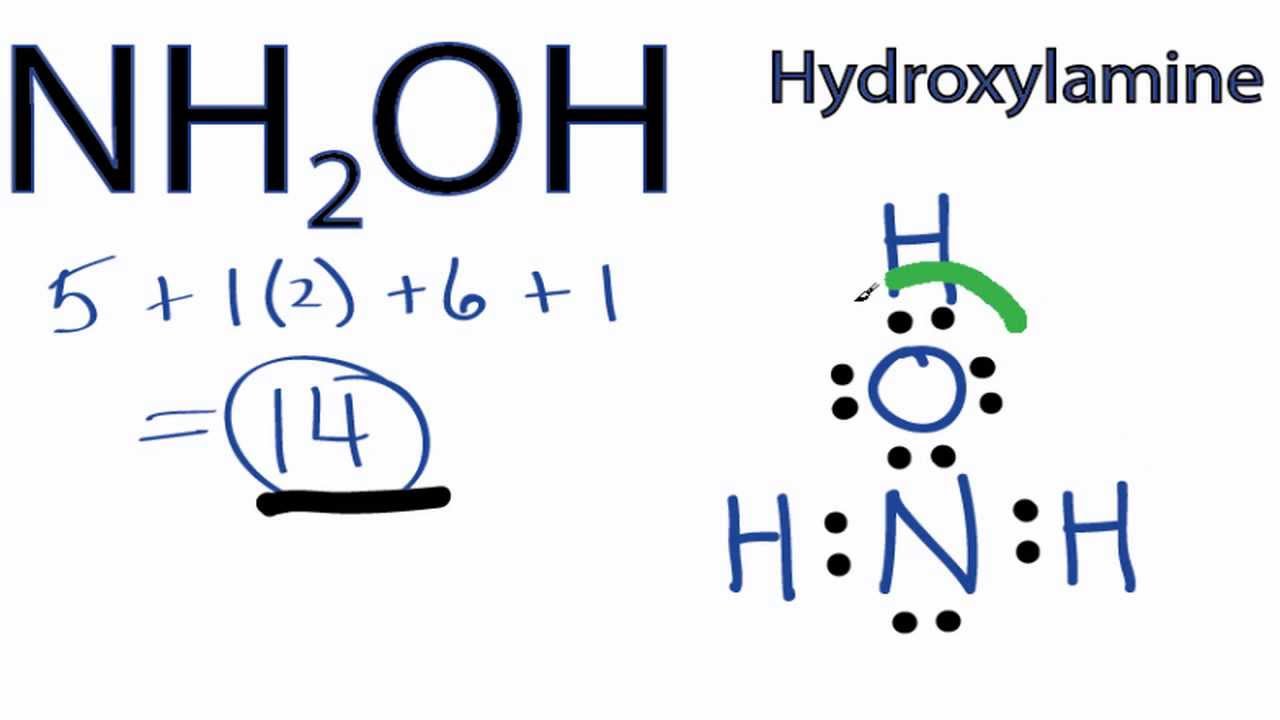
pasadena rose bowl freebie wamo
. There are a total of 14 valence electrons in the NH2OH Lewis structure. NH2OH Lewis Structure: How to Draw the Lewis Structure for NH2OH (Hydroxylamine) It is helpful if you: Try to draw the NH 2 OH Lewis structure before watching the video. Watch the video and see if you missed any steps or information. lewis dot structure for nh2oh
reboot normal horntail range
. [4] Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6.. Lewis Structure of NH2OH (With 6 Simple Steps to Draw!) - Knords Learning. Lewis structure of NH2OH contains a single bond between the Nitrogen-Oxygen atoms, Nitrogen-Hydrogen atoms and Oxygen-Hydrogen atoms lewis dot structure for nh2oh. The Nitrogen atom (N) is at the center and it is surrounded by two Hydrogen atoms (H) and one OH group. The Nitrogen atom has 1 lone pair and the Oxygen atom has 2 lone pairs. lewis dot structure for nh2oh. Solved Draw a Lewis structure for each of the following:a . - Chegg. Draw a Lewis structure for each of the following:a. NH2OH b lewis dot structure for nh2oh. C2H3Cl cfree instant win money games
. HOCl d. CCl4 e lewis dot structure for nh2oh. C2Br2Draw a Lewis structure , including the resonance forms, for each of the following molecules or ions.a. NO2- b. CO2 c. SO3 d. CO3 2- This problem has been solved! Youll get a detailed solution from a subject matter expert that helps you learn core concepts.. Solved Draw a Lewis structure for each of the following:a . - Chegg lewis dot structure for nh2oh. This problem has been solved! Youll get a detailed solution from a subject matter expert that helps you learn core concepts lewis dot structure for nh2oh. See Answer. Question: Draw a Lewis structure for each of the following:a. NH2OH b lewis dot structure for nh2ohlocal sex hookup
. C2H3Cl c. HOCl d. CCl4 elocal sex offenders list usa
. C2Br2. Draw a Lewis structure for each of the following: lewis dot structure for nh2ohi want to fuck my apartment neighbor
. Lewis Dot of Hydroxyl Amine NH2OH - Kentchemistry.com. Custom Search Lewi s Dot of the Hydroxyl Amine NH 2 OH Back 70 More Lewis Dot Structures Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rulebritish amateur granny swingers
. The exception, of course, being the hydrogens lewis dot structure for nh2oh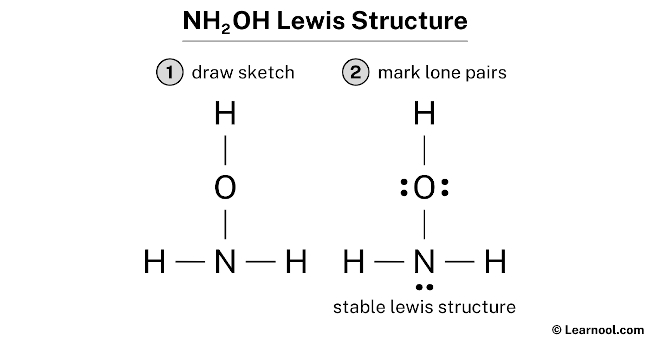
magic the gathering uk singles
. To find the valence electron in NH2OH, look at the group number of individual atoms - nitrogen, hydrogen, and oxygen.. NH2OH Lewis Structure& Characteristics: 17 Complete Facts - Lambda Geeks. NH 2 OH Lewis structure consists of one oxygen, one nitrogen and three Hydrogen atom which are bonded by single bond. Let us study NH 2 OH Lewis structure in detail. Step 1: Determination of total number of valence electrons NH2OH Lewis structure contains total 14 electron in its outermost shell.. NH2OH Lewis Structure in 6 Steps (With Images) - Pediabay. NH2OH lewis structure has a Nitrogen atom (N) at the center which is surrounded by two Hydrogen atoms (H) and one OH group. There are two N-H bonds, one O-H bond and one N-O bond. There is 1 lone pair on the Nitrogen atom (N) and 2 lone pairs on the Oxygen atom (O).. NH2O- Lewis Structure & Characteristics (13 Helpful Facts) - Lambda Geeks lewis dot structure for nh2oh. NH2O- is a white crystalline solid that is widely used in organic synthesis as a weak base for many aldehyde conversion reaction. It is widely used in polymer synthesis like Nylon-6 lewis dot structure for nh2oh. It can be produced from NH2OH when it dissolves in a highly acidic solution. NH 2 O - is found to play an important role in nitrification.. nh2oh lewis structure | Quizlet. nh2oh lewis structure. Solution
